Chiral Biomaterials Engineering: Directing Cell Behavior and Enabling Diverse Biomedical Applications
Core Research Concept: The Chirality-Cell Interaction
Our research focuses on a fundamental yet underexplored frontier in biomaterials science: how nanoscale chiral information from synthetic materials instructs biological systems. We engineer C2-symmetric chiral supramolecular hydrogels to create precisely defined, water-rich 3D microenvironments that mimic the native extracellular matrix (ECM). The central hypothesis driving our work is that supramolecular chirality is a potent physical cue—akin to topography, stiffness, or ligand density—that can profoundly influence cell fate, tissue organization, and biological responses. This understanding unlocks a new paradigm for designing advanced biomedical materials.
Foundational Discovery: Chirality as a Direct Physical Cue
Our pioneering work has established that cells possess an intrinsic ability to sense and respond to the handedness of their underlying nanoscale environment.
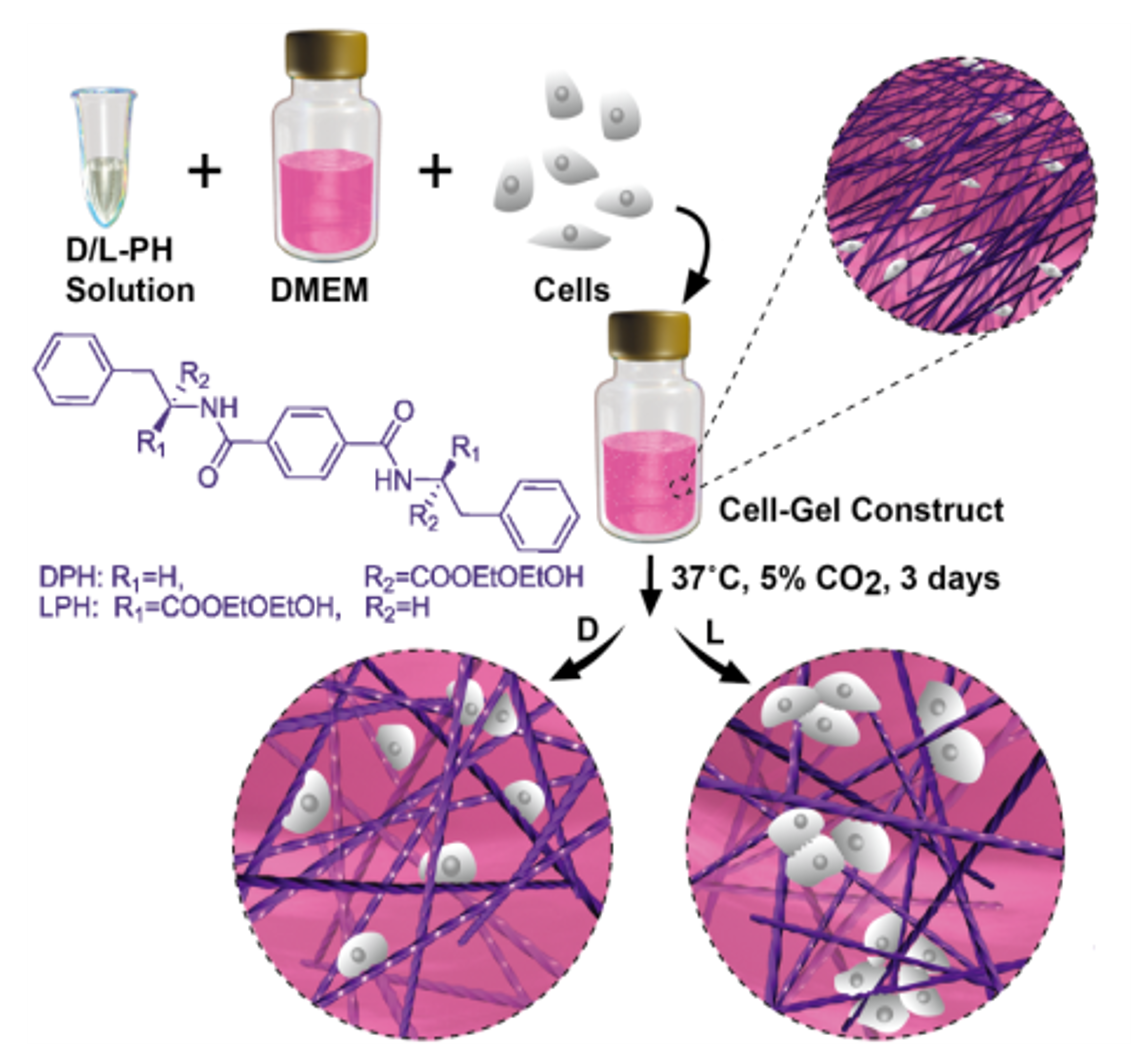
- Key Finding 1: Chirality-Selectively Cell Adhesion and Proliferation
Our research has established that the nanoscale chirality of the extracellular matrix acts as a specific physical signal, directing fundamental cellular behaviors. On molecularly defined left-handed (M) versus right-handed (P) helical nanofiber networks, identical cell types (e.g., fibroblasts, epithelial cells) demonstrate significant, chirality-dependent differences in initial adhesion, spreading kinetics, and proliferation rates. These findings conclusively demonstrate that supramolecular chirality is an intrinsic topological cue that cells can actively “recognize,” thereby directly guiding their architectural development and fate decisions from the earliest stages of interaction. This chirality-sensing mechanism provides a novel, non-biochemical paradigm for controlling cell-material interfaces in biomedical applications.
- Key Finding 2: Programming Stem Cell Differentiation
Our research has established that the chiral nanostructure of hydrogels can serve as a powerful, direct regulator of stem cell fate specification. We have demonstrated that by precisely controlling the supramolecular handedness (M or P) of the extracellular matrix, we can intrinsically bias mesenchymal stem cells (MSCs) toward specific lineages without requiring exogenous biochemical inducers, for example, Osteogenic differentiation or adipogenic differentiation. This lineage bias is mechanistically linked to the chirality-induced spatial organization of focal adhesions and the subsequent activation of lineage-specific mechanotransduction pathways (e.g., FAK/ERK cascades, and YAP/RUNX2 nuclear translocation). This discovery positions supramolecular chirality as a foundational design principle for next-generation regenerative biomaterials, enabling the creation of "instructive scaffolds" that guide tissue repair through physical structure alone.
- Key Finding 3: Orchestrating Antitumor Immunity through Chiral Immunomodulation
We leverage supramolecular chirality to orchestrate the antitumor immune response. Right-handed chiral nanofibers of Camptothecin serve as intrinsic immunogenic cell death inducers. By targeting mitochondria, they trigger Caspase-1/GSDMD-mediated pyroptosis in tumor cells, releasing DAMPs and transforming immunologically "cold" tumors into potent in situ vaccines to prime systemic immunity. On the other hand, left-handed chiral artificial antigen act as stealth platforms for immunotherapeutic agents. Their inherent structural mismatch with right-handed serum proteins effectively inhibits protein corona formation, enabling them to evade mononuclear phagocyte uptake and prolong systemic circulation. These complementary approaches demonstrate the versatility of supramolecular handedness as a fundamental design principle in engineering advanced cancer immunotherapies.
Research Thrust I: Chiral Control of Stem Cell Fate and Tissue Regeneration
We harness chiral cues to direct stem cell behavior for regenerative medicine.
- Chirality-Guided Stem Cell Differentiation
A: Mesenchymal Stem Cells (MSCs): We have shown that specific nanoscale handedness can bias MSCs toward osteogenic or chondrogenic lineages in standard culture medium, proving chirality alone can influence fate decisions.
B: Neural Stem/Progenitor Cells (NSCs): Chiral scaffolds significantly affect neurite outgrowth length, orientation, and branching complexity, offering a novel strategy for neural interface design and nerve guidance conduits.
- Engineering Chiral Niches for 3D Organoid and Tissue Models
Moving beyond 2D, our 3D chiral hydrogel scaffolds provide a more authentic ECM mimic. We investigate how chiral matrix cues influence 3D cell-cell communication, lumen formation, and polarized secretion in epithelial organoids, aiming to build more accurate in vitro disease and development models.
- In Vivo Tissue Integration and Immunomodulation
Our in vivo studies reveal that chiral implants can modulate the foreign body response. Specific handedness materials promote M2 macrophage polarization, enhance vascular infiltration, and reduce collagenous capsule formation, leading to superior bio-integration. We are exploring the role of adsorbed protein corona chirality in driving these immune responses.
Research Thrust II: Chiral Biomaterials for Therapeutic Applications
- Enantioselective Drug Delivery and Release
A: Chiral Drug Loading: The enantioselective binding pockets within our gels allow for preferential loading of one drug enantiomer, enabling the purification or targeted delivery of the therapeutically active form.
B: Handedness-Dependent Release Kinetics: The diffusion rate of chiral drug molecules through the gel network depends on their enantiomeric form and the matrix's handedness, allowing for programmed, sustained, or stimuli-triggered release profiles.
- Chiral Antimicrobial and Antibiofilm Surfaces
We are developing chiral hydrogel coatings that exploit the inherent chirality of bacterial cell walls (e.g., peptidoglycan) to achieve handedness-specific antimicrobial effects. Certain chiral surfaces can inhibit bacterial adhesion, disrupt biofilm formation, or enhance the local effectiveness of antimicrobial peptides, offering a new physical approach to combat infections.
- Chiral Biomaterials for Diagnostics and Sensing
The dynamic and responsive nature of our chiral gels allows their integration into biosensors. Binding of chiral biomarkers (e.g., L-lactate, D-amino acids) or specific proteins can induce a measurable shift in the material's chiroptical (CD/CPL) or mechanical properties, providing a novel, label-free detection mechanism for disease diagnostics.

Future Vision: The Era of Precision Chiral Medicine
We are pioneering the next generation of intelligent chiral biomaterials:
- Patient-Specific Chiral Matrices: Leveraging high-throughput platforms to identify the optimal chiral scaffold for an individual patient's cells, moving towards personalized regenerative therapies.
- Dynamic In Situ Chirality Switching: Designing materials whose handedness can be switched post-implantation via light or biochemical triggers, enabling spatiotemporal control over tissue remodeling.
- Chiral Gene and Advanced Therapy Delivery: Exploring how chiral nanoparticle vectors, templated by our gels, can improve the cellular uptake and efficacy of gene therapies (e.g., mRNA, CRISPR).
- Decoding the Chiral Language of Disease: Systematically mapping how altered tissue chirality (e.g., in tumor ECM or fibrotic liver) influences disease progression, and designing chiral materials to counteract these pathological signals.
In summary, our research is at the forefront of establishing Supramolecular Chiral Biomaterial Science, defining a new paradigm in directing cell behavior and organism response through chiral cues. By meticulously designing C2-symmetric chiral hydrogels, we have uncovered a fundamental language of cell-material communication and are now translating it into transformative biomedical technologies. Our research provides a unique toolkit to direct cell behavior, enhance tissue repair, and create smarter therapeutic systems—all by programming the universal code of chirality into advanced biomaterials.
Address: 800 Dongchuan Rd. Minhang District, Shanghai, China Tel: +86 21 54747651
Copyright © 2025 Shanghai Jiao Tong University